International atomic energy agency mcqs international atomic energy agency quiz answers pdf to study general knowledge online course learn international organizations multiple choice questions and answers mcqs international atomic energy agency quiz questions and answers for job assessment test.
Atomic energy test mcqs.
During an atomic explosion energy released is due to.
Conversion of mechanical energy into nuclear.
For your better entry test preparation and better chemistry mcqs preparation in this section we are going to post atomic structure mcqs.
Nuclear energy levels depend on attractive and repulsive forces.
Past papers of atomic energy latest mcqs sample model papers of atomic energy download past papers of atomic energy sr.
Conversion of chemical energy into heat energy.
Solved past papers of atomic energy test pattern syllabus of atomic energy mcqs papers of paec duration.
Show all questions compared to the charge and mass of a proton an electron has.
Electron energy levels have greater.
Latest mcqs sample model papers pakistan atomic energy commission paec chascent and kinpoe all sample papers general chemical electrical materials metallurgy mechatronics computer engineering download free must prepare now.
Nuclear energy levels are an order of one hundred times as great as electron energy levels.
Conversion of protons into neutrons.
You can skip questions if you would like and come back to them.
Candidate is required to attempt one written examination.
An atom is a small part of an element that takes part in chemical reactions.
How to motivate yourself to change your behavior.
International atomic energy agency mcqs quiz online pdf book download.
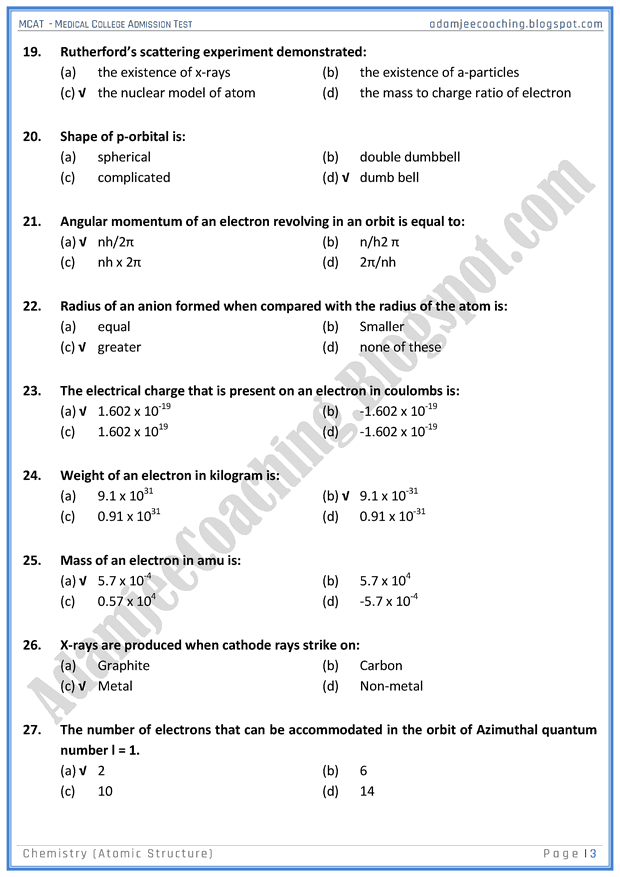
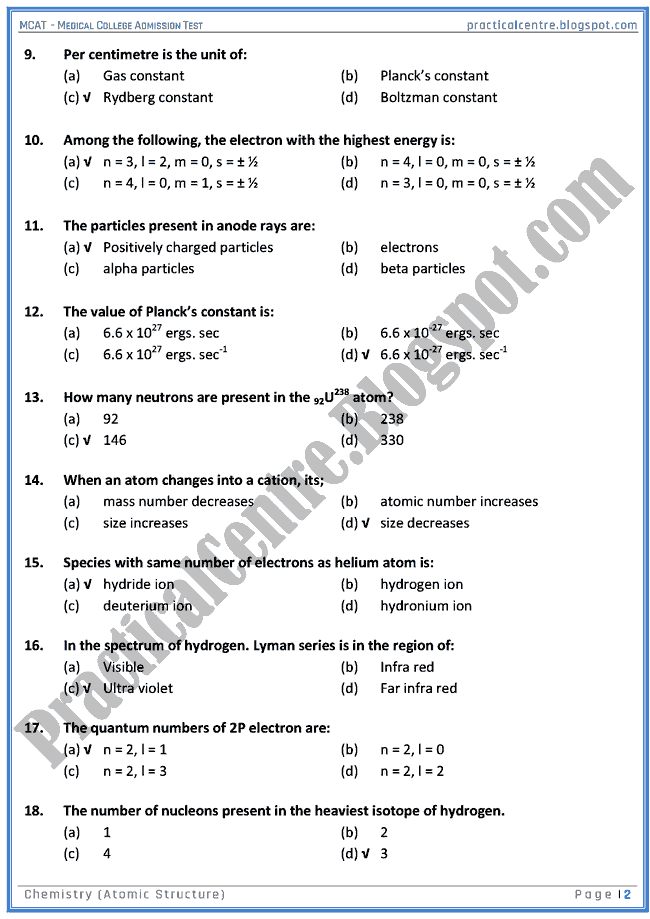
Atomic structure multiple choice questions quiz.
Past papers of pakistan atomic energy commission sample test mcqs will help you alot to solve your queries written test questions.
Choose your answers to the questions and click next to see the next set of questions.
What is the mass number of an atom which contains 28 protons 28 electrons and 34 neutrons.
Past papers of subjects 1 general knowledge 2 chemical technology 3 civil technology 4 computer technology 5 electrical technology.
An ion with 5 protons 6 neutrons and a charge of 3 has an atomic number of.
Electron energy levels depend on the interaction between neutrons and electrons.
Atomic energy chapter exam instructions.
Electrons are the smallest of the.